Botox® (onabotulinumtoxinA) is well known for its ability to help conceal the visible signs of ageing. But Botox, and related products, can also be used as a preventive treatment for chronic migraine. They work to gradually decrease the frequency and severity of migraine attacks and for many people it’s an effective treatment that significantly improves their quality of life.
What are botulinum neurotoxins?
Botox is a purified and highly dilute neurotoxin produced by the Clostridium botulinum bacterium. It’s part of a group of neurotoxins that can cause botulism, a severe illness that causes muscle weakness and paralysis, but in its purified form it’s used both medically and cosmetically.¹
Botox is the brand name of onabotulinumtoxinA which is made by the pharmaceutical company AbbVie (formerly Allergan). Other botulinum products are also used for medical treatment, such as Dysport® (abobotulinumtoxinA, made by Ipsen) and Xeomin® (incobotulinumtoxinA, made by Merz Pharmaceuticals). All of these are classed as a form of Botulinum toxin type A.
How do botulinum neurotoxins work?
Botulinum toxins are used cosmetically to reduce fine lines and wrinkles by blocking the nerve signal to muscles, causing the muscle to relax. However, muscle relaxation is not how it works to reduce migraine attacks.
Botulinum toxins affect the sensory nerves involved in pain pathways, blocking the release of neurotransmitters (brain chemicals) involved in migraine pain, such as calcitonin gene-related peptide (CGRP). Through injections at specific sites under the skin around the face and neck, the toxin blocks pain signals and over time, can help to decrease the brain’s sensitivity and dull down its hyper-reaction to migraine triggers.²’³
The path to migraine use
Botox was first used in the 1970s by ophthalmologists to treat eye conditions such as blepharospasm (an involuntary blinking or eyelid spasm) and squint. Since then, it’s been approved to treat other medical conditions including bladder dysfunction, muscle contractions and excessive armpit sweating.
In 1998, an American plastic surgeon reported some of his patients receiving cosmetic Botox experienced an improvement in their migraine attacks after treatment.³’⁴
Encouraged by other anecdotal evidence, researchers began to formally study the efficacy, safety and tolerability of Botox for chronic migraine (headache on 15 days or more per month for three months or more) through clinical trials. The most notable trials were the Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy clinical program, or PREEMPT study.
The PREEMPT study involved two double-blind, randomised, placebo-controlled clinical trials (the gold standard of clinical trials) involving 1,384 people who fulfilled the trial criteria of chronic migraine. Half the participants received Botox injections and the other half received a placebo.
The results from the clinical trials were combined and the study concluded treating chronic migraine with Botox reduced the number of migraine days and reduced headache-related disability.⁵
From PREEMPT, the Food and Drug Administration (FDA) approved Botox for use in chronic migraine in 2010. Botox has only been approved for chronic migraine as current evidence does not support its use in episodic migraine or tension type headache.³
Injection sites for chronic migraine differ from the sites used cosmetically. A specific protocol for chronic migraine was developed and tested extensively over 10 years to discover the most effective injection sites and dose. A single treatment using this protocol involves 31 injections around the forehead, sides and back of the head, and the neck, using 155 units of botulinum toxin.
Ongoing evidence for the usefulness of Botox for chronic migraine
Further prospective and retrospective studies support the results of the PREEMPT study. The COMPEL study, which began in 2011, included 716 patients with an average of 22 headache days per month. The participants received 155 units of Botox at the 31 injection sites from the established migraine protocol every 12 weeks for 2 years. Just over half the participants completed the study and by weeks 60 and 108, there was a significant reduction in headache days reported.⁶
A systematic review published in 2022 collated data from seven studies on the real-world effectiveness of Botox used for chronic migraine. This found an average reduction of 10 headache days/month and a reduction of 7 days when acute headache pain medication was required. Around half experienced at least a 50% reduction in migraine days. These changes were seen at 24 weeks and persisted for a year of follow-up.⁷
In a study from 2025 comparing Botox with eptinezumab, one of the new anti-CGRP medications, both were equally effective in treating chronic migraine at 3 and 6 months.⁸
Accessing botulinum toxin treatment in the public health system in New Zealand
In New Zealand, Botox was approved by Medsafe in 1991, Dysport in 1992 and Xeomin in 2014. Medsafe approval means that these medications can be sold and marketed in NZ. For these to be funded in the public health system, they must be approved by Pharmac. Botox has been approved by Pharmac for use in chronic migraine prevention (but Dysport is only indicated for treatment of muscle spasms and excessive sweating). However, these are only available and funded from the Hospital Medicines List.
The Hospital Medicines List includes medicines that are funded for use within public hospitals and some specialist services but must be prescribed or administered by hospital staff as part of hospital treatment. Patients don’t pay for these medicines as they’re covered by the hospital’s funding – that is, through Health New Zealand/Te Whatu Ora. As of July 2025, the cost of a 100U vial of Botox on the Hospital Medicines List was $467.50.
In contrast, other preventive migraine medications (like Emgality, Aquipta, amitriptyline and beta blockers) are on the Community Schedule, which includes medicines prescribed by GPs, specialists, or other authorised prescribers which are dispensed in the community by retail pharmacies. For these medicines, patients typically pay a prescription charge (currently around $5 per prescription, with some exemptions), but the medicines themselves are funded directly by Pharmac through the pharmaceutical budget.
Because Botox is a hospital medicine, Te Whatu Ora decides how much it will spend on Botox as part of its overall budget. Anecdotally, very few hospitals in New Zealand allocate any funding or resources to administering Botox for chronic migraine. We have tried to find out more through an Official Information Act request in 2024, but Te Whatu Ora was unable to provide information about how much (if any) Botoxᵀᴹ was used in its hospitals for the treatment of migraine.
As we said at the time:
“This is another example of how a system can’t improve when it can’t or won’t measure what it is or is not doing. If Te Whatu Ora don’t measure a problem, can they then ignore it? Why this is not given as a treatment for chronic migraine in most public hospitals, despite it being approved by Pharmac for this indication?”
Accessing botulinum toxin treatment in the private health system in New Zealand
There are two issues with accessing botulinum toxin treatment in the private health system. The first is the cost, which is around $1,200 per session, repeated every three months. The second is finding an experienced practitioner who knows how to deliver botulinum toxin according to the PREEMPT protocol. It’s not a matter of injecting the toxin into random sites around the forehead and it’s not the same as treatment for wrinkles. If you’re going to pay to get this done, make sure the person administering it follows the approved protocol that has been shown to be effective for chronic migraine treatment.
What to expect during botulinum toxin treatment
Before the first treatment, the clinician administering the toxin should discuss the side effects and risks. This is a good time to ask questions and check they follow the established protocol.
They will use a small needle to inject 0.1ml or 5 units of Botox at each site just under your skin. You may feel a slight sting with each injection. Because Botox comes in 100 unit vials, some clinicians may do a few more injections around areas of tenderness or where you most commonly experience migraine pain, typically in the muscles on the side of the head (temporalis), the muscle at the back of the head (occipitalis), and the trapezius muscles at the neck and shoulders.
The treatment takes about 15–20 minutes and there aren’t any restrictions on driving or other daily activities after treatment.
It’s important to remember that these injections aren’t a quick fix for migraine. It often takes two or three treatments to notice if it’s effective in reducing the frequency or intensity of migraine attacks. Botox treatment is repeated every 12 weeks, which means it may take 6–9 months to notice any difference. Some people have reported improvements after their first treatment. As with many migraine treatments, results are very individualised.
Many people report the benefits of the injections start to wear off after about 10 weeks, and the intensity and frequency of their migraine attacks increase close to their next treatment date.
You don’t need to stop taking other preventive medications with botulinum toxin treatments and you can continue to use acute medications like triptans and non-steroidal anti-inflammatories if you need them.
If after three treatments your migraine attacks haven’t improved, this may not be the right treatment for you.
Side effects and risks with botulinum toxin
Like other medications, there are a few side effects and risks, though most are rare, mild and temporary. Many people’s biggest fear are the needles.
The most common side effect following botulinum toxin treatment is neck pain, reported in about 9% of people during the PREEMPT study.
About 5% of people from the PREEMPT clinical trials reported a migraine attack following treatment. Headache and migraine occurring in the first month after treatment usually decrease with repeat treatments.⁹
Other side effects include:
- drooping eyelids
- pain at the injection site
- muscle pain and weakness
- muscle spasm
- loss of facial movement (forehead and eyebrows)
- skin rash
- itching
- upper respiratory tract infection.⁹
Rare but serious side effects include breathing and swallowing problems and the spread of the toxin.⁹ Botox for chronic migraine is only approved for people aged 18 and older and its safety during pregnancy and breastfeeding is unknown.²
Final thoughts
Botox has been used to treat medical conditions for many decades. It isn’t a cure but it can be very effective as a treatment for chronic migraine on its own or in combination with other preventives. However, in New Zealand this evidence-based treatment is not available to most people with chronic migraine.
The original version of this article was written by Sarah Cahill for Migraine & Headache Australia and reviewed by Dr Bronwyn Jenkins in 2020. This was updated and revised for the New Zealand context in July 2025.
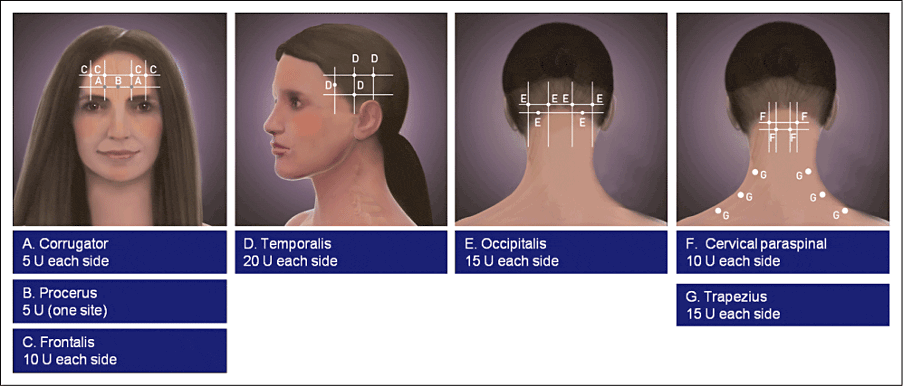
Fixed-site, fixed-dose injection site locations: the (A) corrugators, (B) procerus, (C) frontalis, (D) temporalis, (E) occipitalis, (F) cervical paraspinal, and (G) trapezius muscle injection sites.
Image from: Blumenfeld, A., et al. (2010), Method of Injection of OnabotulinumtoxinA for Chronic Migraine: A Safe, Well-Tolerated, and Effective Treatment Paradigm Based on the PREEMPT Clinical Program. Headache: The Journal of Head and Face Pain, 50: 1406-1418. https://doi.org/10.1111/j.1526-4610.2010.01766.x
References
- The Migraine Trust. (2025). Botox injections for migraine.
- Jenkins, B. (2018). Treatment Spotlight: Botox for Chronic Migraine. Migraine World Summit 2018.
- Blumenfeld, A. (2019). Botox: Separating Fact from Fiction. Migraine World Summit 2019.
- Blumenfeld, A. (2019). Botox for Migraine. MigrainePal
- Dodick, D., Turkel, C., DeGryse, R., et al. (2010). OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program, Headache, 50(6), 921-936. https://doi.org/10.1111/j.1526-4610.2010.01678
- Blumenfeld, A., Stark, R., Freeman, M., et al. (2018). Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study, The Journal of Headache and Pain, 19(13).
- Lanteri-Minet M, Ducros A, Francois C, Olewinska E, Nikodem M, Dupont-Benjamin L. Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: A meta-analysis on 10 years of real-world data. Cephalalgia. 2022;42(14):1543-1564. doi:10.1177/03331024221123058
- Scuteri D, Pagliaro M, Iannacchero R, Trimboli M, Lawrence GW, Bagetta G, Corasaniti MT. Comparing eptinezumab with onabotulinumtoxinA in the treatment of chronic migraine: a real-world evidence study. J Headache Pain. 2025 Jul 14;26(1):159. doi: 10.1186/s10194-025-02106-z.
- AbbVie. BOTOX® Botulinum Toxin Type A injection. Consumer Medicine Information. (2025). NZ CMI v16, DS v18. Available from Medsafe New Zealand.